New research findings present a potential alternative to using creams or ointments on the scalp.
Roflumilast foam was successful in reducing psoriasis and itch after eight weeks in a cohort of plaque psoriasis patients.
Treating scalp psoriasis is challenging, with the associated pruritus having a significant impact on quality of life and the patient’s hair impacting the effectiveness and application of topical creams and ointments. Early trials of other topical formulations, such as foams, suggest these approaches are effective and well tolerated.
The evidence base for foam-based treatments in psoriasis has now increased, courtesy of the results of the ARRECTOR phase 3 trial published in JAMA Dermatology.
As part of the new research, once-daily application of roflumilast – a selective phosphodiesterase 4 inhibitor – more than doubled the proportion of patients successfully reducing both scalp and body psoriasis symptoms compared to a placebo foam.
“These results demonstrate that once-daily roflumilast foam, 0.3%, may provide a monotherapy topical treatment for patients with psoriasis of the scalp and body,” the researchers concluded.
Four hundred and thirty-two patients with plaque psoriasis affecting their scalp and body with a Scalp-Investigator Global Assessment score of at least 3 (moderate) as well as a score of at least 2 (mild) on the rest of the Body-Investigator Global Assessment score were randomised to receive 0.3% roflumilast foam or placebo in a 2:1 fashion. Participants were instructed to apply the foam to all affected body areas.
After eight weeks of treatment, a greater proportion of patients in the roflumilast group achieved the joint primary end points of S-IGA and B-IGA success – a score of 0 (clear), or a score of 1 (almost clear) with at least a two-point improvement from baseline – compared to the control group.
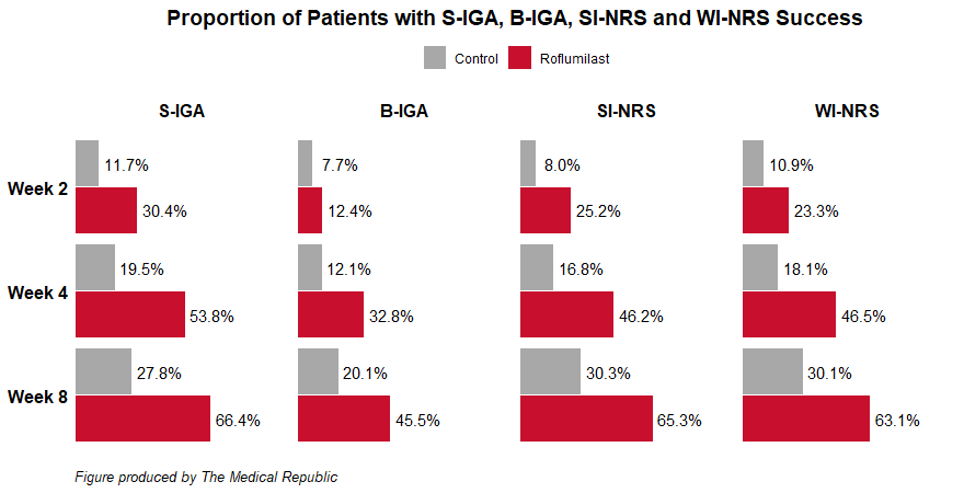
Roflumilast treatment was associated with higher success rates with respect to self-reported pruritus (first measured after 24 hours), where success was defined as a minimum of a four-point improvement in patients with scores of 4 or more on the Scalp Itch-Numeric Rating Scale and the Worst Itch Numeric-Rating Scale.
The researchers felt the improvements in pruritus reported in the immediate post-treatment window also contributed to patient adherence.
Related
“Early improvement in patient-reported outcomes is advantageous because patient satisfaction with therapy can enhance adherence to treatment, particularly when the areas affected by psoriasis are difficult to treat,” they noted.
“Foam formulations are intended to make application to the scalp and other hair bearing areas easier; in the current trial, success in both investigator- and patient-reported outcomes supports the use of the foam formulation.”
A greater proportion of patients treated with roflumilast also achieved at least a 75% improvement in the Psoriasis Scalp Severity Index (71% versus 31%) and the Psoriasis Area and Severity Index (50% versus 17%) after eight weeks compared to patients who received the placebo.
Treatment-related adverse events occurred in less than 6% of patients in each group, while less than 2% of patients in each group discontinued treatment as a result of adverse events.
Roflumilast is far more potent – somewhere between 25- and 300-plus fold more – than other PDE4 inhibitors used in the treatment of dermatological conditions, ultimately amplifying its downstream effects on inflammatory processes.
“Inhibition of PDE4 by roflumilast modulates inflammatory cytokines, many of which are implicated in the pathophysiologic mechanism of inflammatory skin diseases, including psoriasis,” the researchers wrote.
“This modulation decreases expression of key proinflammatory cytokines, including type 1 (interferon gamma, tumour necrosis factor alpha), type 2 (interleukin-4) and type 17 (IL-17, IL-23), and increases anti-inflammatory cytokines, such as IL-10.
“Furthermore, roflumilast inhibits sensory neuron activation, thereby reducing itch sensation, and normalises keratinocyte activation and differentiation, potentially mitigating epidermal barrier dysfunction.”
The FDA approved the use of roflumilast foam in patients with plaque psoriasis over the age of 12 in 2022.





