Clinical trials confirm TNFi therapy, the gold standard treatment for established AS, is also effective for nr-AxSpA Axial spondyloarthritis (axSpA) is a chronic inflammatory disease affecting the axial skeleton and characterised by inflammation of the sacroiliac joints and spine. Patients with axSpA experience chronic back pain and spinal stiffness as well as a reduction in […]
Clinical trials confirm TNFi therapy, the gold standard treatment for established AS, is also effective for nr-AxSpA
Axial spondyloarthritis (axSpA) is a chronic inflammatory disease affecting the axial skeleton and characterised by inflammation of the sacroiliac joints and spine.
Patients with axSpA experience chronic back pain and spinal stiffness as well as a reduction in mobility and quality of life.1 It is a condition with a broad spectrum of clinical manifestations, laboratory abnormalities, and imaging features.2
Historically, a clinical condition is first identified in its most advanced stage when the manifestations are most obvious. The most advanced stage of axSpA is ankylosing spondylitis (AS),2 a condition which was first recognised and named around 1900.1
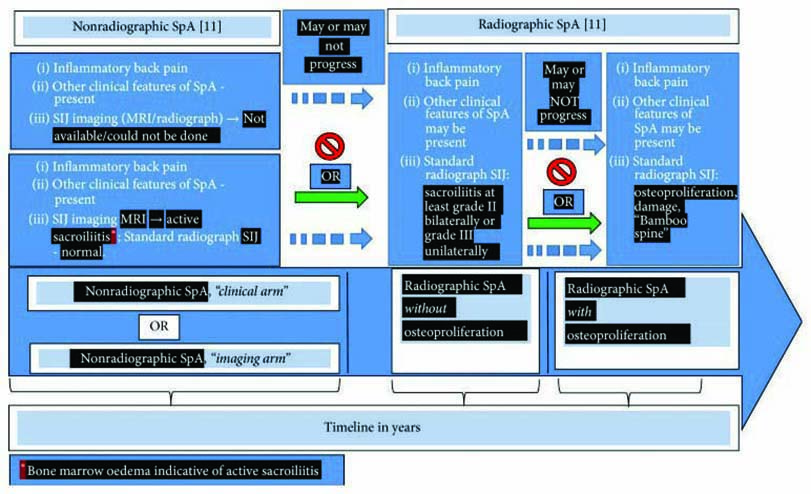
By the 1930s, the use of radiography was able to show structural changes and establish that the disease starts in the sacroiliac joints.1
Thanks to the more recent advent of magnetic resonance imaging (MRI) it has been possible to identify patients at an even earlier stage of the disease. These patients have non-radiographic axial spondyloarthritis (nr-AxSpA), before structural changes in the sacroiliac joints have been detected.1
DISEASE CONTINUUM
Expert opinions suggest nr-AxSpA and AS (the radiographic form of axSpA) are considered now as two consecutive stages of one disease.3 Patients with nr-AxSpA may or may not eventually develop structural damage. However, radiographic sacroiliitis may take years to develop, if at all, complicating identification and delaying management of patients who may have earlier stages but do not present with evident signs of damage in the sacroiliac joints.4
Roughly half of patients presenting with nr-AxSpA will, over the space of a decade, progress to develop AS.5 It may slowly progress with visible radiographic damage showing little or minimal osteoproliferation. Or it may rapidly progress, causing damage and osteoproliferation with the formation of syndesmophytes and subsequent curvature of the spine. In some individuals, however, the disease may be arrested at an early stage without further progression and damage.2
RATIO OF nr-AxSpA TO AS
Most of the available data for nr-AxSpA prevalence are from studies performed in patients with suspected axSpA, but without a clear diagnosis, who were then referred to rheumatologists. Nr-AxSpA or AS was then diagnosed for the first time. These studies indicate that the proportion of nr-AxSpA patients among those with newly diagnosed axSpA can be expected to be between 23% and 80%, depending on the symptom duration, selection criteria, and other parameters, such as availability and interpretation of MRIs.1
Interestingly, some of the studies showed that radiographic sacroiliitis may already be present in 20-30% of patients after only two to three years of symptom duration.1 While there is a predominance of males among those with radiographic sacroiliitis, the proportion of men and women is equal or may even show female preponderance among those without radiographic sacroiliitis.2
DISEASE BURDEN
The German Spondyloarthropathy Inception Cohort (GESPIC) has shown that in patients with nr-AxSpA, the disease burden, as determined by severity of symptoms (measured by the disease activity index the Bath Ankylosing Spondylitis Disease Activity Index [BASDAI], seriousness of global pain and night pain, patient’s global assessment of disease activity, intensity of treatment, response to treatment, and quality of life), does not differ from the patients with radiographic sacroiliitis.2
DIAGNOSIS/SYMPTOMS
Nr-AxSpA has a similar spectrum of clinical features to AS. These include inflammatory back pain, peripheral arthritis affecting large joints in the lower segment of the body with prominent asymmetry and tarsitis, characteristic extraarticular features consisting of episodes of acute anterior uveitis, prominent enthesitis and dactylitis.2 Patients with AS and nr-AxSpA are similar regarding human leukocyte antigen (HLA)-B27 positivity, signs of peripheral inflammation, disease activity indices, and patient-reported outcomes (PROs). Cohort studies have demonstrated that C-reactive protein [CRP] and erythrocyte sedimentation rate [ESR], and especially a disease activity score incorporating CRP or ESR (i.e., the Ankylosing Spondylitis Disease Activity Score), were associated with radiographic spinal progression.4
However, there is limited data available to determine whether and which patients with nr-AxSpA will develop structural changes in the sacroiliac joints and experience progression. Although the similarities and differences between nr-AxSpA and AS may suggest that, for some patients, these classifications represent early and later stages of axial SpA, it is not known whether this distinction is clinically relevant or warrants different treatment strategies.4
CURRENT APPROACHES
As with the treatment of AS, management of nr-AxSpA is best performed by a multidisciplinary team. It is of particular importance to engage with radiologists to ensure they understand what is required for accurately reading MRI scans of inflammation of the sacroiliac joint. Teams also include allied health professionals such as specialist rheumatology nurses, nurse practitioners and physiotherapists.4
TNFi TREATMENT
Clinical trials have confirmed that TNFi therapy, the gold standard treatment for established AS, is also effective in treating
nr-AxSpA.
This is particularly true for cases that are of more recent onset (< three years symptom duration), with elevated ESR or CRP levels, or a positive MRI scan providing objective evidence of inflammation.5 In such cases, the treatment response is similar to that seen with established AS, where TNFi treatment is highly effective.5 A long term two-year follow-up study with TNFi therapy demonstrated that early treatment may prevent radiographic damage and be associated with low disease activity or remission.6
In Australia, SIMPONI and etanercept are TNFis indicated for the treatment of nr-AxSpA in adults with active non-radiographic axial spondyloarthritis with objective signs of inflammation as indicated by elevated C-reactive protein (CRP) and/or magnetic resonance imaging (MRI) evidence, who have had an inadequate response to, or are intolerant to, nonsteroidal anti-inflammatory drugs (NSAIDs).7 Recently the PBAC made a positive recommendation for the SIMPONI to be included on the PBS for the treatment of nr-AxSpA. SIMPONI has shown a rapid reduction in the signs and symptoms of nr-AxSpA and the effect was sustained over 16 weeks.8 Over 70% of patients achieved an ASAS20 response at the study end compared to 40% with placebo.8
Treatment effects were also significant for key secondary efficacy measures, including BASDAI 50 (p<0.0001), ASAS partial remission (p=0.0136) and SPARCC MRI SI score (p<0.0001).8
Editorial created by Rheumatology Republic and sponsored by Janssen-Cilag Pty Ltd. Refer to the Product Information before prescribing, available from www.janssen.com.au/Simponi_PI.
References:
1.
Sieper & van der Heijde: //www.ncbi.nlm.nih.gov/pubmed/23233285
2.
Malaviya: // www.ncbi.nlm.nih.gov/pubmed/28555158
3.
Poddubnyy: //www.ncbi.nlm.nih.gov/pmc/articles/PMC3582305/
4.
Mease et al: // www.ncbi.nlm.nih.gov/pubmed/29409123
5.
Brown & Bradbury: //www.mja.com.au/journal/2017/206/5/new-approaches-ankylosing-spondylitis
6.
Cantarini et al : //journals.lww.com/md-journal/Fulltext/2015/07050/Effectiveness_of_Adalimumab_in_Non_radiographic.11.aspx
7.
SIMPONI Product Information (28 November 2017). Available at: https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2011-PI-03025-3&d=201809061016933
8.
Sieper et al.: //www.ncbi.nlm.nih.gov/pubmed/26139307